How verbal ‘opt out’ was an acceptable alternative to informed consent in a low risk neonatal trial: findings from the neoGASTRIC Trial

Published on Thursday, 11 December 2025
Authors: Professor Kerry Woolfall, University of Liverpool & Dr Tracy Mitchell, University of Liverpool
Background
Research is needed to inform evidence-based treatments for babies in neonatal intensive care, yet seeking informed consent for a study is challenging when a mother has just given birth and her baby is critically ill. Understandably, parents can be distressed and anxious about their newborn and may not feel able to consider their babies involvement in research at that point in time.
Some international neonatal randomised trials have used an 'opt-out' approach to consent as a way to reduce potential burden and improve recruitment. Such trials involve automatic enrolment into a study after parents have been provided the opportunity to 'opt-out' of their baby's involvement. This alternative to informed consent has been approved by ethics committees as the studies were seen as low risk, comparing treatments that would usually be given to babies in neonatal units and do not need to be given in an emergency, so there is some time (e.g. > 48 hours) between admission and randomisation.
The neoGASTRIC trial is the largest individually randomised controlled trial enrolling preterm babies. Involving >60 UK and Australian recruiting sites, this pragmatic unmasked parallel group trial evaluates whether not routinely measuring gastric residual volumes (stomach contents) in preterm infants reduces the time taken for a baby to reach full enteral feeds, without increasing necrotising enterocolitis. As part of an embedded process evaluation, we explored parent and staff views on the use of an opt-out approach to inform ongoing trial recruitment, staff training, and future neonatal trials.
Who was involved?
To explore experiences of the opt out process we used questionnaires, interviews and focus groups. A total of 85 parents (59 mothers, 26 fathers) approached about neoGASTRIC in four participating hospitals completed a questionnaire. Of these, 7/85 (8%) opted-out of their baby's involvement in neoGASTRIC; opt-out rate in the wider trial at the same point (152/2043, 7.4%). We also interviewed 15 parents and conducted focus groups with two focus groups involving 18 nurses from three sites.
What did we find?
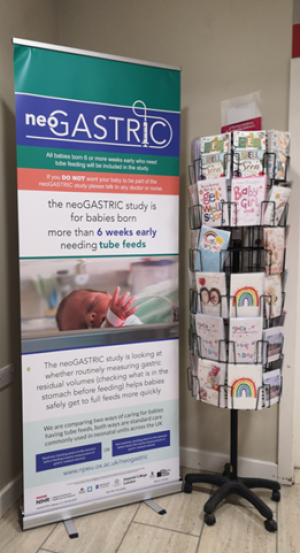
Parents and staff supported opt-out consent in neoGASTRIC as interventions were viewed as low-risk and non-invasive. Parents appreciated an appropriately timed research conversation. Only 21% noticed study information banners/posters (as shown in the picture) highlighting the importance of verbal information and written information sheets to inform decision making.
How and when the opt-out process took place varied at different hospitals in terms of when information was provided and when babies were randomised to the study. We found that women approached during labour or within hours of birth reported feeling overwhelmed and lacking capacity to consider research.
I thought it'd be more of a priority to make sure he was, like, sort of, stable, before they'd ask those types of questions (P12, father, interview).
Parents who felt they were approached at a convenient time (e.g. within days of birth rather than hours) appeared to have better recall of the discussion and felt the timing was appropriate when
I was a little bit more with it” (P7, mother, interview):
By speaking to us on day 3 meant I could understand and support the study (P61, mother, questionnaire).
Parents who opted their baby out stated that they did so due to uncertainties about the intervention and potential impact on their child, or use of data. None raised concerns about full informed consent not being sought.
What does this mean for neoGASTRIC and future trials?
An appropriately timed verbal opt-out approach to consent was seen acceptable as proportionate in the neonatal context in a low-risk trial comparing different accepted clinical, non-pharmaceutical, practices. Where interventions are time-critical, Research Without Prior Consent (also known as deferred consent) should be considered. Findings informed neoGASTRIC and will guide approaches to consent in this setting. Guidance for sites to inform ongoing recruitment are shown in the box below.
Recommendations for future trials:
1. Assess the acceptability of an opt out approach with families at the pre-trial stage
2. If using an opt out approach, ensure posters and/or banners are displayed in prominent places.
3. Tailor the recruitment approach for each family. It may be OK to pause randomisation to discuss the study and give parents more time to read about the trial.
4. Consider the timing of approach. Do not approach parents during labour or too soon after birth; Provide trial information at least a day after birth, or antenatally in the days preceding labour.
Please access the full published paper here if you would like to find out more: Parent and practitioner experiences of opt-out consent in neonatal intensive care: a mixed methods study within a trial | ADC Fetal & Neonatal Edition




