Health professionals
Background
Routine measurement of gastric residual volume is the practice of regularly aspirating the entire stomach contents in order to assess the volume and colour of the gastric 'aspirate'. The use of gastric feeding tubes is standard of care for preterm babies below approximately 34 gestational weeks. Although the routine measurement of gastric residual volumes is widely practised, it is poorly evidenced and there is wide variation in how gastric residual volumes are interpreted and how they influence feeding decisions. Evidence suggests that gastric aspiration inaccurately measures gastric residual fluid volume. It is also uncomfortable for the baby and there are concerns that it is potentially harmful to the baby in delaying time to reach full milk feeds. At the moment we do not know whether measuring gastric residual volumes is beneficial or harmful or has no effect.
Study Aim
To test whether avoiding the routine measurement of gastric residual volumes in preterm infants reduces the time taken for a baby to reach full enteral feeds without increasing harms.
Study Population
Preterm infants (born less than 34+0 gestational weeks+days) admitted to participating neonatal units in the United Kingdom and Australia.
Trial design
Infants will be randomised as soon as possible after consent using a 1:1 allocation ratio to either:
NO ROUTINE MEASUREMENT OF GASTRIC RESIDUAL VOLUMES
or
ROUTINE, UP TO 6 HOURLY, MEASUREMENT OF GASTRIC RESIDUAL VOLUMES
Inclusion Criteria
- Gestational age at birth less than 34+0 gestational weeks+days (up to and including 33+6 gestational weeks+days)
- Nasogastric or orogastric tube in place
Exclusion Criteria
- Infant has received more than 15 ml/kg/day of milk for more than 24 hours
- Gastrointestinal surgical condition (including suspected necrotising enterocolitis and focal intestinal perforation) prior to randomisation
- Major congenital abnormalities
- No realistic prospect of survival
- A parent has opted out of infant's participation in neoGASTRIC
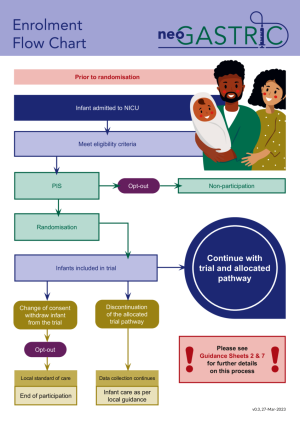
For full information about the neoGASTRIC trial, please see the trial protocol and enrolment flowchart.