Study Findings
Baby-OSCAR trial – Short and long term outcome results
(Short term results published NEJM 25th Jan 2024)
(Long term results published eClinicalMedicine 20th August 2025)
Why did we do this trial?
Patent ductus arteriosus (PDA) is an extra blood vessel found in babies before and just after birth. For most babies, the blood vessel will shrink and close on its own shortly after birth but the blood vessel is likely to stay open for longer in babies who are born early (prematurely). This can lead to too much blood flowing into the baby's lungs and can cause health complications like heart failure and bronchopulmonary dysplasia (dependence on oxygen or breathing support). It can also increase the risk of the development of an infection of the intestines, called necrotising enterocolitis.
What did we do?
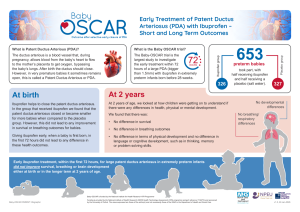
The Baby-OSCAR trial, was carried out in 32 neonatal intensive care units across the UK and coordinated by the National Perinatal Epidemiology Unit Clinical Trials Unit. It looked at whether or not giving ibuprofen in the first 72 hours after birth could help reduce the risk of death or developing bronchopulmonary dysplasia in extremely premature babies born with a large patent ductus arteriosus.
653 babies born between 23 weeks and 28 weeks of pregnancy took part in the trial. All of them had a large patent ductus arteriosus (more than 1.5mm with significant flow) confirmed by a chest ultrasound scan (echocardiogram). A total of 326 babies were randomly assigned to receive ibuprofen and 327 babies to receive a placebo (saltwater solution). The trial looked at the health of babies during their initial hospital stay at birth to understand whether the treatment made a difference to survival and breathing problems. At two years of age, the children were followed up again to understand if there were any longer-term differences in health, physical or mental development.
What did we find?
Results from the Baby-OSCAR trial have shown that treatment with ibuprofen in the first 72 hours after birth in extremely preterm babies born with patent ductus arteriosus, does not improve survival rates or reduce bronchopulmonary dysplasia at 36 weeks post menstrual age. The treatment with ibuprofen, however did significantly reduce the number of babies with an open or large PDA at 3 weeks of age. At two years of age, we found that there was no difference in survival or breathing outcomes. We also found no difference in terms of physical development and no difference in language or cognitive development such as thinking, memory or problem solving skills.
Key findings:
- Treatment with ibuprofen within 72 hours of birth did not reduce the risk of death or bronchopulmonary dysplasia when compared with placebo;
- 69.2% of babies assigned to ibuprofen either died or developed bronchopulmonary dysplasia compared with 64.1% babies who were assigned to the placebo;
- Among babies who survived to 36 weeks post-menstrual age (the number of weeks since the mother's last period), 64.2% assigned to ibuprofen and 59.3% assigned to placebo developed bronchopulmonary dysplasia;
- A single course of ibuprofen given within 72 hours of birth resulted in a closed or smaller patent ductus arteriosus at 3 weeks of age in 55.5% of babies in the ibuprofen group;
- There was no evidence that treatment with ibuprofen resulted in any additional serious health complications.
- There was no difference in survival, neurodevelopment or breathing outcomes at 2 years of age.
- At two years of age survival without moderate to severe neurodevelopmental impairment was 53% in those that received ibuprofen compared to 51.9% for babies that received the placebo.
What does this mean for extremely premature babies born with a large patent ductus arteriosus?
Baby-OSCAR is the largest trial globally to assess treatment for patent ductus arteriosus in 25 years. The results of the trial provide an important contribution towards the treatments for patent ductus arteriosus and will help neonatologists and people caring for extremely premature babies to make informed decisions about the risks and benefits of treatment.
The trial team would like to thank all babies and their parents for participating in this trial.
The trial was funded by the National Institute for Health and Care Research Health Technology Assessment programme and sponsored by the University of Oxford.
The short term study findings of the Baby-OSCAR trial are available on the NEJM website. The long term results can be found on the Science Direct website.