Frequently Asked Questions
These FAQs address:
- Reviewing
- Reporting
- Membership of the local PMRT review group
- Using the PMRT
- Reports generated by the PMRT
- Governance
If you have a question which is not addressed here or in the Guidance documentation please contact us either using the 'contact us' facility within the tool or by emailing us at: mbrrace.support@npeu.ox.ac.uk
Reviewing
- Which perinatal deaths can we review using the PMRT?
-
- Late fetal losses (also called late miscarriages) where the baby is born between 22+0 and 23+6 weeks of pregnancy showing no signs of life, irrespective of when the death occurred, or if the gestation is not known, where the baby is over 500g;
-
All stillbirths where the baby is born from 24+0 weeks gestation showing no signs of life, or if the gestation is not known, where the baby is over 500g. For the rare stillbirths which are unattended at home and where no antenatal care had been received, the review should focus on any postnatal and bereavement care provided;
- All neonatal deaths where the baby is born alive from 22+0 weeks of pregnancy but dies up to 28 days after birth, or if the gestation is not known, where the baby is over 500g;
-
All neonatal deaths where the baby dies in the community up to 28 days after birth or later, who have not received any neonatal care, should nevertheless be reviewed to ensure that the baby was indeed well at discharge and that appropriate bereavement care was provided;
- Post-neonatal deaths where the baby is born alive from 22+0 but dies after 28 days following neonatal care; the baby may be receiving planned palliative care elsewhere (including at home) when they die.
-
Which perinatal deaths should we not use the PMRT to review?
-
-
Termination of pregnancy at any gestation;
-
Babies with brain injury who survive.
Trusts in England should refer to the Maternity Incentive Scheme (MIS) run by NHS Resolution to check the requirements of the reviews that are included in Safety Action 1.
-
-
How do we conduct a review when care was provided in more than one trust/health board?
-
- To ensure that the whole pathway of care is reviewed, and that a single coherent report of the review findings is generated, the ideal is that a joint review is carried out by all the units involved in providing care for the mother and baby. The unit where the baby died is responsible for initiating the PMRT review.
- We appreciate that organising joint meetings may be complex and not possible in all instances, but the use of video conferencing for joint discussions could be considered.
-
To ensure that any issues with care identified are 'owned' by the appropriate trust/health board, the review should be 'assigned' (see below for details of the assignment function) prior to a joint meeting so that the other trust/health board can review their aspects of care ahead of the joint discussion.
-
In the event that it is not possible to organise a joint review it is better that care is reviewed separately than not at all and that all units review the part of the care pathway they were involved in providing. To support this the 'assignment' feature is part of the PMRT. Assignment allows the unit initiating the review to 'assign' the review to the other unit(s) involved in care to enable them to review the care they provided. Following their review, they reassign the PMRT back to the unit which initiated the review. This also ensures that any issues with care are 'owned' by the unit that generates them and importantly a single report coherent report is generated for discussion at the follow-up meeting with parents.
Reporting
- Do I need to report eligible cases to MBRRACE-UK as well as using the PMRT to review the same cases?
-
- Yes, you should report eligible deaths through the MBRRACE online reporting system. You should then complete the surveillance form, if required, and commence a review with the PMRT for relevant deaths.
- The eligibility criteria for surveillance and review are different. The system will tell you when surveillance or review is required.
-
Information in the notification is populated automatically into the surveillance form and review. Additional information in the surveillance form which is common to the PMRT, can be imported into the review, but not vice versa. We therefore recommend that you complete the surveillance form before starting the review. To reduce further duplication of effort we are extending this cross-population of information.
- Why do we need to report to MBRRACE-UK and use the PMRT?
-
- The MBRRACE-UK online reporting form is a data collection tool for national surveillance. The PMRT has been designed as a review tool to assist units in completing a structured, standardised and thorough review of the whole pathway of care; it is therefore important that both are used as they fulfil different functions.
Membership of the local PMRT review group
Who should be involved in our PMRT review group?
-
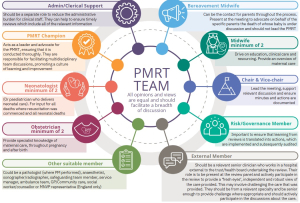
- PMRT reviews must be carried out by multidisciplinary groups. We have developed a recommended group composition which is listed in the PMRT guidance document. The guidance document is available on the PMRT implementation support page. We have also created a team infographic which you can see below or download here.
- To be multidisciplinary, the team conducting the review should include at least one and preferably two of each of the professionals involved in the care of pregnant women and their babies. Ideally, the team should also include an external member (see below).
- Bereavement care staff (midwives and nurses) should form part of the review team to provide their expertise in reviewing the bereavement and follow-up care, and advocate for the parents. Unless they have been specifically employed to do so, it should not be the responsibility of bereavement care staff to run the reviews, chair the panels nor provide administrative support.
- Do we have to have an external member as part of the group?
-
- Ideally the multidisciplinary review team should include an external member(s).
- Parents are reassured by the presence of an external panel member, as otherwise the review may be seen as the trust/health board 'marking their own homework' without any external scrutiny.
- External panel member(s) should be relevant senior clinicians who are currently practicing clinically and work in a hospital external to the trust/health board undertaking the review and external to any trust/health board involved in the care at any stage.
- Their role is to be present at the review panel and actively participate in the review, to provide a 'fresh pair of eyes' and an independent and robust view of the care provided. This may involve challenging the care provided. They should be from relevant specialities (you may require more than one, dependent upon the details of the death and care involved), should be senior enough to provide challenge where appropriate, and should actively participate in the discussions about the care.
- If multiple trusts/health boards were involved in the review due to their involvement in the care, none of their staff members are 'external' panel members. Since they cannot provide an independent assessment of the care, they should not be listed as 'external' members in the participant list.
- Although the MNVP member (England only) may not be employed directly by the trust, they should not be regarded as, nor documented as, an 'external' member. They are present to represent the wider parent voice and not to act as an external panel member.
- To ensure that the involvement of external members of the review panel is recorded accurately, the 'participant' feature in the PMRT system has been updated to make this easier.
- Being an external panel member is also of benefit to the individuals fulfilling this role since exposure to other ways of working can provide valuable continuing professional development (CPD) and can be recorded as such.
How might we organise having an external(s) as part of our MDT panel meetings?
Some trusts/health boards have difficulty organising external member(s) for their multi-disciplinary team (MDT) PMRT panel meetings. This is a particular issue for some trusts in England where there is a requirement for an external to be present for at least 50% of all MDT panel meetings as part of the Maternity Incentive Scheme.
- Some trusts in England used the additional Ockenden monies to fund time in obstetric job plans, which has been maintained since those funds ran out;
- Monthly perinatal mortality reports to their trust Board have been used by one trust to make the case for additional funding to support additional PAs in job plans;
- Several neonatal operational delivery networks (ODNs) are providing funding to ensure neonatologists attend panels as externals;
- One ODN obtained funding from the Specialist Commissioner to fund extra neonatologist sessions for attendance at panels.
- The LMNS undertook a survey of trusts to identify staff and their general availability. With this they have generated a spreadsheet and use this to act as matchmakers with the staff in trusts organising panels.
- This works best when panel meetings are scheduled well ahead, for example, for a year at a time, with regular meetings. The regular meetings can always be cancelled if there are few or no deaths to review, or the review(s) needs to be postponed whilst further information is sought.
- The staff member in a trust in charge of convening their meetings contacts the LMNS with the dates and times of their meeting and the LMNS match a suitable external based on the specifics of the death. For example, for a mother and baby who received complex obstetric and neonatal care, both an external obstetrician and neonatologist will be scheduled for the panel.
Using the PMRT
- We have identified additional 'issues' that are separate to those automatically generated by the tool what should we do?
-
- The PMRT generates 'issues' based on the answers provided to the questions about the care provided. It then asks you to assess whether the issues contributed to the outcome, the underlying cause for each one, and whether action to improve care is needed regardless of the contribution to the outcome.
- You may identify additional 'issues' that are not identified by the tool; you should use the 'add custom issue' feature to generate your own issues. You can create as many of these as is necessary.

- How do we review multiple pregnancies when both/all of the babies died?
-
- At present a separate review for each baby will need to be carried out. In the future, we plan to develop feature so that there will be an option to share the common antenatal care details and review. When this feature is enabled the babies will be able to have a common antenatal review but separate intrapartum and postnatal reviews of care will still be required.
- What is the purpose of the 'notes' function?
-
-
The white text boxes on the right side of the screen are organised by different elements of the review (e.g., antenatal, intrapartum). These boxes allow you to type notes about the care during the review, similar to jotting down notes on paper. Any text entered will automatically transfer to the final clinical report, where you can edit the information as needed. The boxes can also be used to document good care, but whether this information should be included in the final report shared with parents should be considered individually.
-
Important: If the review is completed and the report is 'published', but then reopened and re-reviewed, any new notes added will not be automatically copied into the updated report.
-
When writing the report, including after the review has been reopened, you can view all the original notes by clicking on “Click here to see the unmodified narratives”. This includes any new notes added after the review has been reopened, which can then be copied into the revised report.
-
- We are having difficulty finding the contributory factor we want to allocate?
-
-
The contributory factors are included as a three stage drop down menu representing the three levels of the National Patient Safety Contributory Factors Classification Framework. You might find it helpful to print out the Framework in the table or fish bone format so that they can be looked at as a whole, separately during the meeting. This will make it easier to find the correct stem to identify the relevant contributory factors within the tool.
-
- How do we save the review?
-
- The PMRT automatically saves throughout the review process. The save status is displayed in the bottom right of the screen. The review for each baby can be opened and amended as many times as needed prior to completion.
- Once the review process is complete and the 'Complete Review' button is clicked the form is validated. If there are any validation errors you can correct them where appropriate and then accept any outstanding 'errors' by providing a reason to ignore them. Once validation is complete and the session is closed the clinical report generation is automatically started and the review can no longer be edited. If the review is in the report writing stage you can reopen the review yourself and once published, you can request for a review to be reopened by getting in touch with the PMRT team: mbrrace.support@npeu.ox.ac.uk
- Why is the review so long when we know the issue only occurred in one part of the care?
-
- The PMRT was specifically designed to support the review of the whole pathway of care; this prevents pre-judging the reason why the baby died and therefore only reviewing some elements of care, which could lead to vital information being missed. We know from the confidential enquiry findings that significant issues with care are missed when only specific aspects of care are considered in isolation. It is also important to note that all reviews provide the potential to identify significant issues with care that, whilst they may not have contributed to this particular death, nevertheless need addressing to improve care for all women and babies.
- What if the questions in the tool don't enable us to review the care properly?
-
-
There may be questions which we have omitted or responses to questions which don't include relevant answers. If you identify such omissions in the tool please let us know so that we can modify and improve the tool to meet your needs. You can do this within the PMRT using the 'contact us' function or by emailing us at: mbrrace.support@npeu.ox.ac.uk
-
- How do I print the clinical review report?
-
- The clinical review report can be printed once it has been 'Published' by pressing the 'Download' button. This will download a PDF file, which can be printed. You are also able to download your draft report before final publication.
Reports generated by the PMRT
- What should we give to the parents, as the report seems very medical?
-
- We suggest that you use the clinical report as the basis for the discussion with parents but that you write a letter for the parents' using appropriate language after you have met with them for their follow-up appointment. This letter should include a summary of your discussion with them and the review findings. You can ask the parents if they would like a copy of the clinical report, but should explain that this may be quite technical.
- Can we have a summary of the cases that have been reviewed?
-
-
The PMRT enables registered users to specify a period of time for which they require a report and download a 'summary report'. This report provides an overview of the number of deaths within that period and their review status. For deaths where the report has been published, the report also summarises issues with care, and outlines required actions for improvement.
-
- Will there be reports available?
-
-
National annual reports available and we provide a report each year, exploring national data. You can find them on the reports page here.
-
Governance
- How can we incorporate the PMRT within our governance process?
-
- It is important to ensure that the PMRT forms part of the governance structure within your organisation. This can be supported by developing a standard operating procedure and sign off level requirements for particular deaths. Developing a relationship with your governance team is also essential for identifying and ensuring that actions generated from the reviews are implemented and monitored. We strongly recommend that your multidisciplinary team includes members from your risk and governance team.
- How does the PMRT fit within the serious incident (SI) process?
-
- If a stillbirth or neonatal death is identified through your hospital governance process as a serious incident (SI) then the completion of the PMRT will form a substantial part of an SI investigation. The PMRT will enable a thorough, structured review of the care provided. This may then need to be supplemented by further documentation as per the hospital/national governance requirements to complete an SI investigation. This approach will avoid potential duplication of effort reviewing essentially the same information in a broadly similar process. An SI investigation should not delay the PMRT review.
- Can I upload case documents to use in the PMRT?
-
- The PMRT does not have the facility to store additional documents, although it will retain copies of the clinical review reports created using the tool.


